Transferring regeneration-associated genes from easy organisms to advanced animals could possibly be a option to fight getting old and improve longevity.
In a examine that has a touch of sci-fi about it, researchers from the University of Tokyo’s Graduate School of Pharmaceutical Sciences have reported on the intriguing chance of extending lifespan by gene switch. By borrowing genes from species identified for his or her exceptional regenerative skills – comparable to jellyfish and flatworms – the scientists aimed to analyze whether or not these genes may improve the regenerative capacities and longevity of different organisms. Whereas full regeneration remains to be extra prone to be discovered on an episode of Physician Who, the analysis of those different medical doctors marks a major step in direction of understanding how genes linked to regeneration may doubtlessly impression getting old processes.
Longevity.Expertise: Repairing harm is a therapeutic method we regularly talk about, however the geroscientific implications of analysis into regeneration are profound, opening new doorways to novel therapies that might delay and even reverse features of getting old. By understanding and leveraging the organic mechanisms that drive getting old, longevity researchers can develop interventions that enhance healthspan, and by specializing in genes related to excessive regenerative capability, new methods for sustaining stem cell perform and tissue homeostasis will be discovered, enhancing the standard of life in getting old populations.
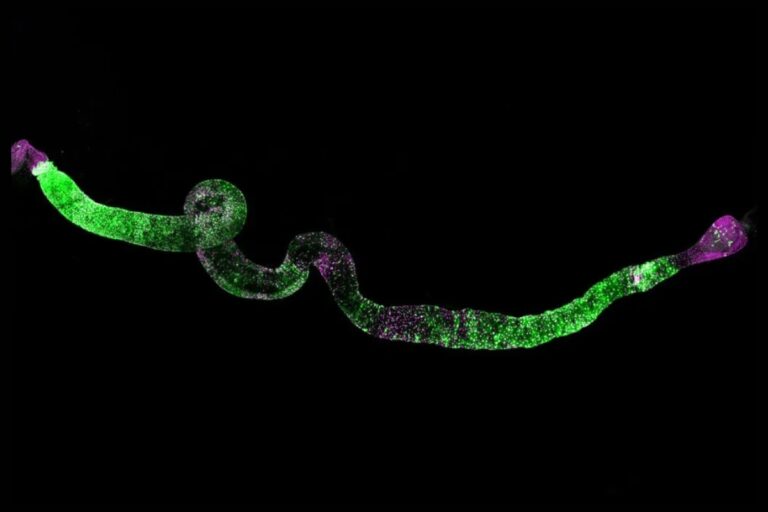
On this examine, the researchers launched extremely regenerative species-specific JmjC domain-encoding genes (HRJDs) into the widespread fruit fly, Drosophila melanogaster, a mannequin organism with restricted regenerative skills. These HRJDs are a gaggle of genes distinctive to animals able to whole-body regeneration [1].
“In animals able to whole-body regeneration, comparable to flatworms and jellyfish, particular genes could assist permit regeneration and preserve long-term stem cell capabilities,” defined Affiliate Professor Yuichiro Nakajima, a number one researcher within the examine. “Conversely, mammals and bugs, which have restricted regenerative skills, could have misplaced these genes throughout evolution [2].”
The objective was to watch whether or not reintroducing these genes may have a useful impact on regeneration and getting old processes in a species not sometimes identified for its regenerative prowess.
The outcomes had been each stunning and promising; whereas the researchers initially hoped that the fruit flies may exhibit enhanced tissue regeneration, the fact was considerably completely different. The introduction of HRJDs didn’t spur new tissue progress in injured flies, however an surprising but important discovering was noticed within the intestinal stem cells of getting old flies.


Hiroki Nagai, an knowledgeable in fruit fly intestines, found that HRJDs promoted better division of intestinal stem cells whereas suppressing the differentiation errors that sometimes happen as these cells age [1].
“HRJDs promoted better intestinal stem cell division, while additionally suppressing intestinal cells that had been mis-differentiating, or going fallacious in aged flies,” Nakajima famous [2]. This twin motion is especially noteworthy because it contrasts with conventional strategies, comparable to using antibiotics, which could suppress the problematic cells but in addition hinder useful stem cell exercise.
“For that reason, HRJDs had a measurable impact on the lifespans of fruit flies, which opens the door, or a minimum of offers clues, for the event of latest anti-aging methods.” he added. “In spite of everything, human and bug intestines have surprisingly a lot in widespread on a mobile degree [2].”


The implications of those findings are manifold – not solely do they counsel a possible pathway for enhancing the well being span of organisms, however in addition they elevate questions concerning the elementary mechanisms underlying regeneration and getting old. The examine revealed that the expression of HRJDs prolonged the lifespan of the fruit flies beneath non-regenerative circumstances, particularly by enhancing the proliferative exercise of intestinal stem cells whereas sustaining their differentiation constancy [1]. This amelioration of age-related decline in intestine barrier capabilities is an important perception, because it factors to a possible methodology for preserving the integrity of important tissues and organs in getting old animals.
Nonetheless, as with every pioneering analysis, there are each challenges and limitations. The getting old course of in fruit flies, although comparatively fast in contrast with different animals, nonetheless spans roughly two months. This timeframe offered logistical challenges for the researchers, who needed to juggle this examine with different analysis. Furthermore, whereas the present findings are promising, the exact molecular mechanisms by which HRJDs exert their results stay largely unexplored.


“Particulars of the molecular workings of HRJDs are nonetheless unresolved. And it’s unclear whether or not they work alone or together with another part,” Nakajima admitted, highlighting the preliminary nature of this analysis.
Nonetheless, the examine offers a helpful framework for future investigations. As Nakajima factors out: “That is simply the beginning of the journey, however we all know now that our modified fruit flies can function a helpful useful resource to uncover unprecedented mechanisms of stem cell rejuvenation sooner or later [2].”
On condition that human and bug intestines share notable similarities on the mobile degree, the potential for translating these findings into human therapies is an thrilling, albeit distant, prospect. The prospect of introducing regeneration-associated genes to fight getting old and promote wholesome lifespan is an attractive avenue of analysis that will in the future result in advances in regenerative medication which can be very a lot science reality.
Pictures ©2024 Yuichiro Nakajima CC-BY-ND
[1] https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-024-01956-4
[2] https://www.u-tokyo.ac.jp/focus/en/press/z0508_00365.html