A placebo-controlled Part 1/2 trial performed in East Shanghai has discovered that administering umbilical cord-derived mesenchymal stem cells reduces frailty in older people.
Stem cells towards frailty
These researchers start by defining frailty as “a state of heightened vulnerability to potential stressors as a consequence of discount in physiological reserves throughout a number of techniques” [1]. This vulnerability destroys the energy and endurance of older individuals, exhausting their stamina and drastically growing their dangers of dying and incapacity, and a metric has been decided to measure this [2]. Nonetheless, whereas vitamin dietary supplements might assist individuals with dietary deficiencies, there are not any medically permitted medication to deal with frailty [3].
As stem cell exhaustion has been pinpointed as a reason behind frailty [4], alternative stem cells have been investigated as a doable therapy. Specifically, mesenchymal stem cells (MSCs), that are naturally interested in harm websites [5], look like essentially the most promising. MSCs have a number of potential sources for derivation [6], and former trials have been performed to deal with frailty through the use of MSCs derived from bone marrow (BM-MSCs), with optimistic outcomes [7, 8].
This examine, nonetheless, was performed on stem cells that have been initially derived from the human umbilical twine (HUC-MSCs). These cells are simple to mass produce [9], have been efficiently clinically examined towards different ailments comparable to coronary heart failure [10] and arthritis [11], and battle irritation [12]. This, nonetheless, is the primary trial of HUC-MSCs for frailty.
Frail adults solely
All individuals needed to meet three standards: to be between the ages of 60 and 80, to attain between 1 and 4 on the Fried frailty scale [2], and to be anticipated to dwell one other yr. A big number of co-morbidities have been screened out, comparable to uncontrolled diabetes, critical cardiovascular issues, infections, and viral ailments. This was a double-blinded trial from which 80 potential candidates have been excluded. 15 sufferers obtained placebo, and 15 obtained MSCs, for six months.
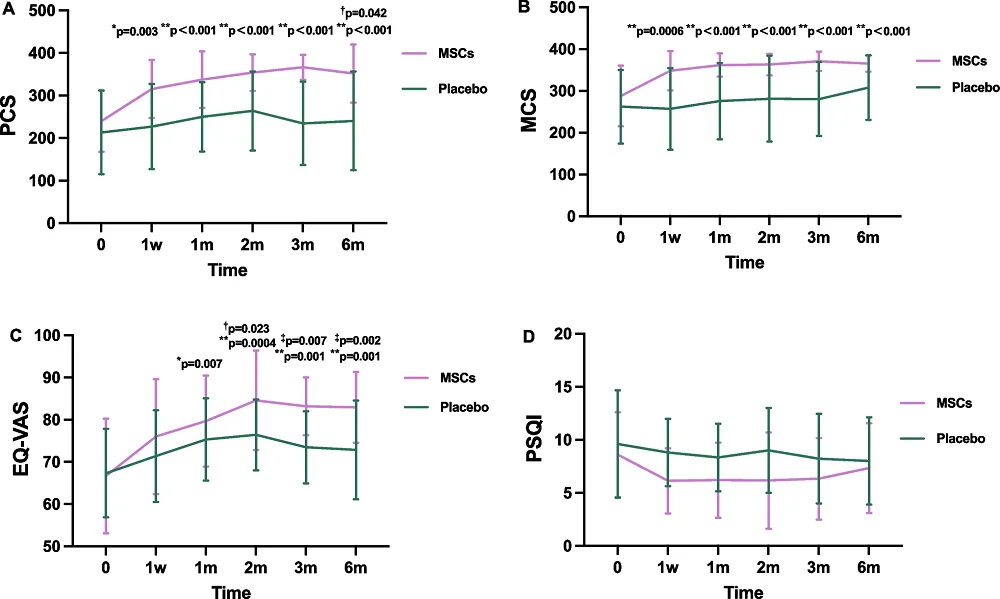
This examine measured bodily efficiency by testing grip energy, the timed up-and-go take a look at, strolling velocity, and the flexibility to face up and sit again down. Inflammatory cytokines comparable to interleukins have been additionally measured, and sleep high quality, high quality of life, and psychological well being have been additionally assessed.
Solely good important results
There have been no important hostile results. Three individuals had suffered from illnesses through the trial, two of which have been within the placebo group and the third of which had dizziness not associated to the MSCs.
Bodily perform, the first endpoint of the examine, was strongly affected by the MSCs. Even with solely 30 complete individuals, not all of which participated in each evaluation, the researchers have been capable of get hold of, towards baseline, a p-value of .003 after just one week of therapy and p-values below .001 for 1 and 6 months. Towards placebo, the p-value on the finish of the 6-month examine was .042.
There have been doable results on psychological well being and sleep high quality however these could possibly be statistically attributed to the placebo impact. Nonetheless, the therapy improved complete high quality of life with a p-value of 0.002 towards placebo on the finish of the examine.
Cytokines had much less clear results; the placebo group spiked in TNF-α and IL-17 at 6 months whereas the MSC group didn’t.
Whereas not the entire endpoints have been hit, this examine was towards frailty, and it’s clear from these outcomes that MSCs have helpful impacts on frailty in human beings. Nonetheless, this examine was performed in a single nation amongst 30 individuals. Additional work, with a bigger pattern dimension and extra testing websites, will must be performed to find out if these outcomes maintain up below additional scrutiny.
Literature
[1] Clegg, A., Younger, J., Iliffe, S., Rikkert, M. O., & Rockwood, Okay. (2013). Frailty in aged individuals. The lancet, 381(9868), 752-762.
[2] Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., … & McBurnie, M. A. (2001). Frailty in older adults: proof for a phenotype. The Journals of Gerontology Sequence A: Organic Sciences and Medical Sciences, 56(3), M146-M157.
[3] Dent, E., Morley, J. E., Cruz-Jentoft, A. J., Woodhouse, L., Rodríguez-Mañas, L., Fried, L. P., … & Vellas, B. (2019). Bodily frailty: ICFSR worldwide scientific apply pointers for identification and administration. The Journal of diet, well being and growing older, 23(9), 771-787.
[4] Schulman, I. H., Balkan, W., & Hare, J. M. (2018). Mesenchymal stem cell remedy for growing older frailty. Frontiers in Diet, 5, 108.
[5] Golpanian, S., Wolf, A., Hatzistergos, Okay. E., & Hare, J. M. (2016). Rebuilding the broken coronary heart: mesenchymal stem cells, cell-based remedy, and engineered coronary heart tissue. Physiological critiques, 96(3), 1127-1168.
[6] Zhang, J., Huang, X., Wang, H., Liu, X., Zhang, T., Wang, Y., & Hu, D. (2015). The challenges and guarantees of allogeneic mesenchymal stem cells to be used as a cell-based remedy. Stem cell analysis & remedy, 6, 1-7.
[7] Golpanian, S., DiFede, D. L., Khan, A., Schulman, I. H., Landin, A. M., Tompkins, B. A., … & Hare, J. M. (2017). Allogeneic human mesenchymal stem cell infusions for growing older frailty. Journals of Gerontology Sequence A: Biomedical Sciences and Medical Sciences, 72(11), 1505-1512.
[8] Tompkins, B. A., DiFede, D. L., Khan, A., Landin, A. M., Schulman, I. H., Pujol, M. V., … & Hare, J. M. (2017). Allogeneic mesenchymal stem cells ameliorate growing older frailty: a section II randomized, double-blind, placebo-controlled scientific trial. Journals of Gerontology Sequence A: Biomedical Sciences and Medical Sciences, 72(11), 1513-1522.
[9] Sarugaser, R., Lickorish, D., Baksh, D., Hosseini, M. M., & Davies, J. E. (2005). Human umbilical twine perivascular (HUCPV) cells: a supply of mesenchymal progenitors. Stem cells, 23(2), 220-229.
[10] Bartolucci, J., Verdugo, F. J., González, P. L., Larrea, R. E., Abarzua, E., Goset, C., … & Khoury, M. (2017). Security and efficacy of the intravenous infusion of umbilical twine mesenchymal stem cells in sufferers with coronary heart failure: a section 1/2 randomized managed trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circulation analysis, 121(10), 1192-1204.
[11] Wang, L., Huang, S., Li, S., Li, M., Shi, J., Bai, W., … & Liu, Y. (2019). Efficacy and security of umbilical twine mesenchymal stem cell remedy for rheumatoid arthritis sufferers: a potential section I/II examine. Drug design, growth and remedy, 4331-4340.
[12] Uccelli, A., Pistoia, V., & Moretta, L. (2007). Mesenchymal stem cells: a brand new technique for immunosuppression?. Tendencies in immunology, 28(5), 219-226.


